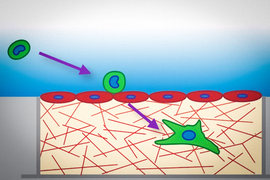
When cancer cells metastasize, they often travel in the bloodstream to a remote tissue or organ, where they then escape by squeezing through the blood vessel wall and entering the site of metastasis. A study from MIT now shows that tumor cells become much softer as they undergo this process.
The findings suggest that drugs that prevent cells from softening could potentially slow or halt metastasis. Metastatic tumors are estimated to be present in about 90 percent of patients who die of cancer.
“We have long thought that if we could identify the barriers that a cancer cell has to overcome to form a metastatic tumor, that new drugs could be found and lives could be saved,” says Roger Kamm, the Cecil and Ida Green Distinguished Professor of Biological and Mechanical Engineering and an author of the study.
MIT graduate student Anya Roberts is the lead author of the paper, which appears today in the Journal of Biomechanics. Giuliano Scarcelli, an associate professor of bioengineering at the University of Maryland, is the senior author. Other MIT authors include Peter So, a professor of mechanical engineering and biological engineering, and Vijay Raj Singh, a research scientist in the Department of Mechanical Engineering.
Squeezing by
After tumor cells enter the blood circulation, they get transported to another location in the body where they can then undergo a process called transendothelial migration. This occurs when cells squeeze between two neighboring endothelial cells (the cells that make up blood vessels), enter the tissue, and begin to multiply. In 2013, Kamm’s lab was first able to explore this process using a microscopic model of the blood capillaries that allowed them to image cancer cells making their way through a blood vessel wall into the surrounding extracellular matrix.
That study and the new paper are both part of an ongoing effort at MIT and elsewhere to study the physical changes that occur in cancer cells when they metastasize. In the new work, the MIT and University of Maryland researchers set out to test their hypothesis that cells become softer during transendothelial migration, making it easier for them to squeeze through small gaps between the endothelial cells.
To explore that possibility, the researchers created a 3D tissue model of the lining of a blood vessel. The model contains a layer of endothelial cells on top of a collagen gel layer that simulates the extracellular matrix. The researchers placed three different types of aggressive, metastatic tumor cells — lung cancer cells, breast cancer cells, and melanoma cells — on the endothelial layer, and measured the cells’ mechanical properties as they passed through the lining.
Many of the existing techniques for measuring cell stiffness, including atomic force microscopy, require physical contact with the cells, which can alter the cells’ mechanical properties. To avoid that kind of interference, the researchers decided to use two optical techniques, which don’t require any contact with the cells being studied and also enable measurements of the nucleus, the stiffest part of the cell interior.
The first of these optical techniques, known as Brillouin confocal microscopy, can reveal how the mechanical properties of a cell change over time in a 3D environment. This technique measures how light scatters when it interacts with density fluctuations within a material, which correlate with the stiffness of the material.
The second technique, known as confocal reflectance quantitative phase microscopy, measures thermal fluctuations of the cell membrane and the nuclear membrane. Softer membranes have larger fluctuations, while stiffer membranes have smaller fluctuations.
Using these two techniques, the researchers found that all of the cancer cell types that they studied became significantly softer as they passed through the wall of the simulated blood vessel. Overall, the lung, skin, and breast cancer cells softened by 30, 20, and 20 percent, respectively. The nuclei of these cells softened by 32, 21, and 25 percent, respectively.
This softening began two to three hours after the cells began their migration (a process also known as extravasation), and the cells were still soft when measured 24 hours later. The researchers suspect that these softened cells may also differ biologically from the cells in the original tumor in ways that make them resistant to conventional chemotherapies.
“This softening may enable these tumor cells to survive, migrate farther into a new tissue location, and build up a secondary metastasis site,” Roberts says.
Disrupting metastasis
It remains unknown what causes the cells to become softer, but the researchers suspect that it may be caused by changes to the structure of chromatin, consisting of DNA and proteins, which is located in the nucleus.
The researchers hope that their work could lead to the development of new drugs that can interfere with cell softening and thus disrupt metastasis.
“There is a chance that some chemotherapeutics may change the nuclear mechanical properties of tumor cells, and soften them,” Roberts says. “It’s a challenging problem, but it’s worth working on. If one could selectively stiffen tumor cells, that might inhibit the formation of a metastasis.”
The research was funded by the National Cancer Institute and the National Science Foundation.