Lead, arsenic, and other heavy metals are increasingly present in water systems around the world due to human activities, such as pesticide use and, more recently, the inadequate disposal of electronic waste. Chronic exposure to even trace levels of these contaminants, at concentrations of parts per billion, can cause debilitating health conditions in pregnant women, children, and other vulnerable populations.
Monitoring water for heavy metals is a formidable task, however, particularly for resource-constrained regions where workers must collect many liters of water and chemically preserve samples before transporting them to distant laboratories for analysis.
To simplify the monitoring process, MIT researchers have developed an approach called SEPSTAT, for solid-phase extraction, preservation, storage, transportation, and analysis of trace contaminants. The method is based on a small, user-friendly device the team developed, which absorbs trace contaminants in water and preserves them in a dry state so the samples can be easily dropped in the mail and shipped to a laboratory for further analysis.
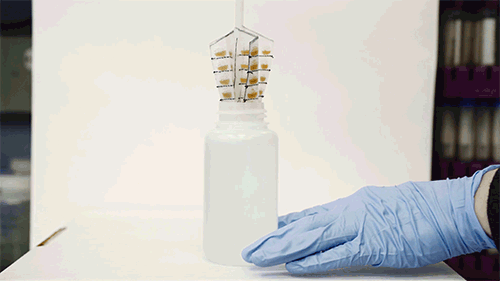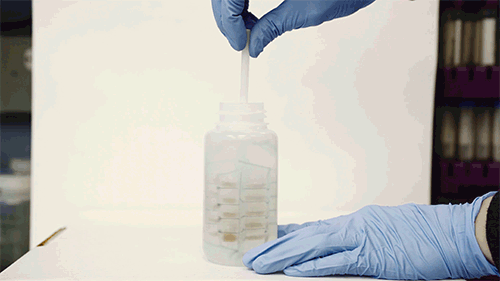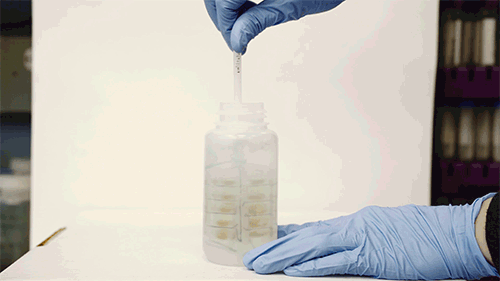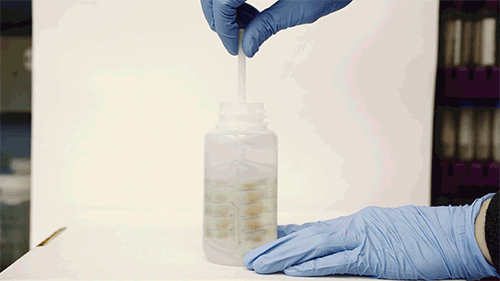
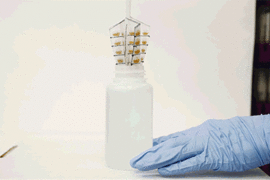
A whisk-like device lined with small pockets filled with gold polymer beads, fits inside a typical sampling bottle, and can be twirled to pick up any metal contaminants in water.
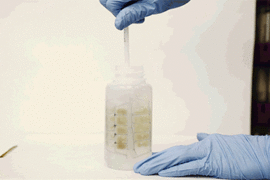
The device resembles a small, flexible propeller, or whisk, which fits inside a typical sampling bottle. When twirled inside the bottle for several minutes, the instrument can absorb most of the trace contaminants in the water sample. A user can either air-dry the device or blot it with a piece of paper, then flatten it and mail it in an envelope to a laboratory, where scientists can dip it in a solution of acid to remove the contaminants and collect them for further analysis in the lab.
“We initially designed this for use in India, but it’s taught me a lot about our own water issues and trace contaminants in the United States,” says device designer Emily Hanhauser, a graduate student in MIT’s Department of Mechanical Engineering. “For instance, someone who has heard about the water crisis in Flint, Michigan, who now wants to know what’s in their water, might one day order something like this online, do the test themselves, and send it to a lab.”
Hanhauser and her colleagues recently published their results in the journal Environmental Science and Technology. Her MIT co-authors are Chintan Vaishnav of the Tata Center for Technology and Design and the MIT Sloan School of Management; John Hart, associate professor of mechanical engineering; and Rohit Karnik, professor of mechanical engineering and associate department head for education, along with Michael Bono of Boston University.
From teabags to whisks
The team originally set out to understand the water monitoring infrastructure in India. Millions of water samples are collected by workers at local laboratories all around the country, which are equipped to perform basic water quality analysis. However, to analyze trace contaminants, workers at these local labs need to chemically preserve large numbers of water samples and transport the vessels, often over hundreds of kilometers, to state capitals, where centralized labs have facilities to properly analyze trace contaminants.
“If you’re collecting a lot of these samples and trying to bring them to a lab, it’s pretty onerous work, and there is a significant transportation barrier,” Hanhauser says.

After the device is pulled out and dried, it can preserve any metal contaminants that it has picked up, for long periods of time. The device can be flattened and mailed to a lab, where the contaminants can be further analyzed.
In looking to streamline the logistics of water monitoring, she and her colleagues wondered whether they could bypass the need to transport the water, and instead transport the contaminants by themselves, in a dry state.
They eventually found inspiration in dry blood spotting, a simple technique that involves pricking a person’s finger and collecting a drop of blood on a card of cellulose. When dried, the chemicals in the blood are stable and preserved, and the cards can be mailed off for further analysis, avoiding the need to preserve and ship large volumes of blood.
The team started thinking of a similar collection system for heavy metals, and looked through the literature for materials that could both absorb trace contaminants from water and keep them stable when dry.
They eventually settled on ion-exchange resins, a class of material that comes in the form of small polymer beads, several hundreds of microns wide. These beads contain groups of molecules bound to a hydrogen ion. When dipped in water, the hydrogen comes off and can be exchanged with another ion, such as a heavy metal cation, that takes hydrogen’s place on the bead. In this way, the beads can absorb heavy metals and other trace contaminants from water.
The researchers then looked for ways to immerse the beads in water, and first considered a teabag-like design. They filled a mesh-like pocket with beads and dunked it in water they spiked with heavy metals. They found, though, that it took days for the beads to adequately absorb the contaminants if they simply left the teabag in the water. When they stirred the teabag around, turbulence sped the process somewhat, but it still took far too long for the beads, packed into one large teabag, to absorb the contaminants.
Ultimately, Hanhauser found that a handheld stirring design worked best to take up metal contaminants in water within a reasonable amount of time. The device is made from a polymer mesh cut into several propeller-like panels. Within each panel, Hanhauser hand-stitched small pockets, which she filled with polymer beads. She then stitched each panel around a polymer stick to resemble a sort of egg beater or whisk.
Testing the waters
The researchers fabricated several of the devices, then tested them on samples of natural water collected around Boston, including the Charles and Mystic rivers. They spiked the samples with various heavy metal contaminants, such as lead, copper, nickel, and cadmium, then stuck a device in the bottle of each sample, and twirled it around by hand to catch and absorb the contaminants. They then placed the devices on a counter to dry overnight.
To recover the contaminants from the device, they dipped the device in hydrochloric acid. The hydrogen in the solution effectively knocks away any ions attached to the polymer beads, including heavy metals, which can then be collected and analyzed with instruments such as mass spectrometers.
The researchers found that by stirring the device in the water sample, the device was able to absorb and preserve about 94 percent of the metal contaminants in each sample. In their recent trials, they found they could still detect the contaminants and predict their concentrations in the original water samples, with an accuracy range of 10 to 20 percent, even after storing the device in a dry state for up to two years.
With a cost of less than $2, the researchers believe that the device could facilitate transport of samples to centralized laboratories, collection and preservation of samples for future analysis, and acquisition of water quality data in a centralized manner, which, in turn, could help to identify sources of contamination, guide policies, and enable improved water quality management.
The researchers have now partnered with a company in India, in hopes of commercializing the device. Together, their project was recently chosen as one of 26 proposals out of more than 950 to be funded by the Indian government under its Atal New India Challenge program.
This research was funded, in part, by the MIT Abdul Latif Jameel Water and Food Systems Lab, the MIT Tata Center, and the National Science Foundation.