Drugs manufactured by living cells, also called biologics, are one of the fastest-growing segments of the pharmaceutical industry. These drugs, often antibodies or other proteins, are being used to treat cancer, arthritis, and many other diseases.
Monitoring the quality of these drugs has proven challenging, however, because protein production by living cells is much more difficult to control than the synthesis of traditional drugs. Typically these drugs consist of small organic molecules produced by a series of chemical reactions.
MIT engineers have devised a new way to analyze biologics as they are being produced, which could lead to faster and more efficient safety tests for such drugs. The system, based on a series of nanoscale filters, could also be deployed to test drugs immediately before administering them, to ensure they haven’t degraded before reaching the patient.
“Right now there is no mechanism for checking the validity of the protein postrelease,” says Jongyoon Han, an MIT professor of electrical engineering and computer science. “If you have analytics that consume a very small amount of a sample but also provide critical safety information about aggregation and binding, we can think about point-of-care analytics.”
Han is the senior author of the paper, which appears in the May 22 issue of Nature Nanotechnology. The paper’s lead author is MIT postdoc Sung Hee Ko.
A complicated process
Many biologics are produced in “bioreactors” populated by cells that have been engineered to produce large quantities of certain proteins such as antibodies or cytokines (a type of signaling molecule used by the immune system). Some of these protein drugs also require the addition of sugar molecules through a process known as glycosylation.
“Proteins are inherently more complicated than small-molecule drugs. Even if you run the same exact bioreactor process, you may end up with different proteins, with different glycosylation and different activity,” Han says.
Although manufacturers can monitor bioreactor conditions such as temperature and pH, which may warn of potential problems, there is no way to test the quality of the proteins until after production is complete, and that process can take months.
“At the end of that process, you may or may not get a good batch. And if you happen to get a bad batch, this means a lot of waste in overall manufacturing workflow,” Han says.
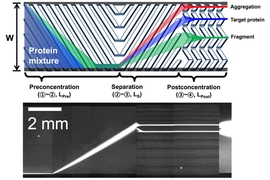
Han believed that nanofilters he had previously developed could be adapted to sort proteins by size as they flow through a tiny channel, which could allow for continuous, automatic monitoring as the proteins are produced. This size information can reveal whether the proteins have clumped together, which is a sign that the protein has lost its original structure.
After proteins enter the nanofilter array device, they are directed to one side of the wall. This narrow line of proteins then encounters a series of slanted filters with tiny pores (15 to 30 nanometers). The pores are designed so that smaller proteins will fit through them easily, while larger proteins will move along the diagonal for some distance before making it through one of the pores. This allows the proteins to be separated based on their size: Smaller proteins stay closer to the side where they started, while larger proteins drift toward the opposite side.
By changing the size of the pores, the researchers can use this system to separate proteins ranging in mass from 20 to hundreds of kilodaltons. This allows them to determine whether the proteins have formed large clumps that could provoke a dangerous immune response in patients.
The researchers tested their device on three proteins: human growth hormone; interferon alpha-2b, a cytokine that is being tested as a cancer drug; and granulocyte-colony stimulating factor (GCSF), which is used to stimulate production of white blood cells.
To demonstrate the device’s ability to reveal protein degradation, the researchers exposed these proteins to harmful conditions such as heat, hydrogen peroxide, and ultraviolet light. Separating the proteins through the nanofilter array device allowed the researchers to accurately determine if they had degraded or not.
Sorting by size can also reveal whether proteins bind to their intended targets. To do this, the researchers mixed the biologics with protein fragments that the drugs are meant to target. If the biologics and protein fragments bind correctly, they form a larger protein with a distinctive size.
Rapid analysis
This nanofluidic system can analyze a small protein sample in 30 to 40 minutes, plus the few hours it takes to prepare the sample. However, the researchers believe they can speed that up by further miniaturizing the device.
“We may be able to do it in tens of minutes, or even a few minutes,” Han says. “If we realize that, we may be able to do real point-of-care checks. That’s the future direction.”
The research was funded by the Defense Advanced Research Projects Agency, SPAWAR Systems Center Pacific, and some authors were supported by a Siebel Fellowship and a Samsung Scholarship.