MIT engineers have devised a way to rapidly test hundreds of different drug-delivery vehicles in living animals, making it easier to discover promising new ways to deliver a class of drugs called biologics, which includes antibodies, peptides, RNA, and DNA, to human patients.
In a study appearing in the journal Integrative Biology, the researchers used this technology to identify materials that can efficiently deliver RNA to zebrafish and also to rodents. This type of high-speed screen could help overcome one of the major bottlenecks in developing disease treatments based on biologics: It is challenging to find safe and effective ways to deliver them.
“Biologics is the fastest growing field in biotech, because it gives you the ability to do highly predictive designs with unique targeting capabilities,” says senior author Mehmet Fatih Yanik, an associate professor of electrical engineering and computer science and biological engineering. “However, delivery of biologics to diseased tissues is challenging, because they are significantly larger and more complex than conventional drugs.”
“By combining this work with our previously published high-throughput screening system, we are able to create a drug-discovery pipeline with efficiency we had never imagined before,” adds Tsung-Yao Chang, a recent MIT PhD recipient and one of the paper’s lead authors.
Peng Shi, a former MIT postdoc who is now an assistant professor at the University of Hong Kong, is the paper’s other lead author.
Fish on the fly
Zebrafish are commonly used to model human diseases, in part because their larvae are transparent, making it easy to see the effects of genetic mutations or drugs.
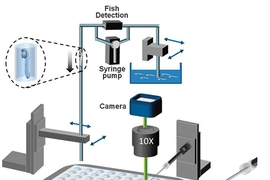
In 2010, Yanik’s team developed a technology for rapidly moving zebrafish larvae to an imaging platform, orienting them correctly, and imaging them. This kind of automated system makes it possible to do large-scale studies because analyzing each larva takes less than 20 seconds, compared with the several minutes it would take for a scientist to evaluate the larvae by hand.
For this study, Yanik’s team developed a new technology to inject RNA carried by nanoparticles called lipidoids, previously designed by Daniel Anderson, an associate professor of chemical engineering, member of the Koch Institute for Integrative Cancer Research and Institute for Medical Engineering and Science, and an author of the new paper. These fatty molecules have shown promise as delivery vehicles for RNA interference, a process that allows disease-causing genes to be turned off with small strands of RNA.
Yanik’s group tested about 100 lipidoids that had not performed well in tests of RNA delivery in cells grown in a lab dish. They designed each lipidoid to carry RNA expressing a fluorescent protein, allowing them to easily track RNA delivery, and injected the lipidoids into the spinal fluid of the zebrafish.
To automate that process, the zebrafish were oriented either laterally or dorsally once they arrived on the viewing platform. Once the larvae were properly aligned, they were immobilized by a hydrogel. Then, the lipidoid-RNA complex was automatically injected, guided by a computer vision algorithm. The system can be adapted to target any organ, and the process takes about 14 seconds per fish.
A few hours after injection, the researchers imaged the zebrafish to see if they displayed any fluorescent protein in the brain, indicating whether the RNA successfully entered the brain tissue, was taken up by the cells, and expressed the desired protein.
The researchers found that several lipidoids that had not performed well in cultured cells did deliver RNA efficiently in the zebrafish model. They next tested six randomly selected best- and worst-performing lipidoids in rats and found that the correlation between performance in rats and in zebrafish was 97 percent, suggesting that zebrafish are a good model for predicting drug-delivery success in mammals.
“The ability to identify useful drug delivery nanoparticles using this miniaturized system holds great potential for accelerating our discovery process,” Anderson says.
“The lipidoid material screen is just an example demonstrated in this article; a similar strategy can be readily extended to other libraries or other organ systems,” Peng adds.
Jeff Karp, an associate professor of medicine at Harvard Medical School who was not part of the research team, says this work is “an excellent example of harnessing a multidisciplinary team to partner complementary technologies for the purpose of solving a unified problem. Yanik and colleagues, who have extensive expertise with high-throughput screening in zebrafish and other small animals, have teamed up with Anderson et al., who are leading experts in RNA delivery, to create a new platform for rapidly screening biologics and methods to deliver them. This approach should have utility across multiple disease areas.”
New leads
The researchers are now using what they learned about the most successful lipidoids identified in this study to try to design even better possibilities. “If we can pick up certain design features from the screens, it can guide us to design larger combinatorial libraries based on these leads,” Yanik says.
Yanik’s lab is currently using this technology to find delivery vehicles that can carry biologics across the blood-brain barrier — a very selective barrier that makes it difficult for drugs or other large molecules to enter the brain through the bloodstream.
The research was funded by the National Institutes of Health, the Packard Award in Science and Engineering, Sanofi Pharmaceuticals, Foxconn Technology Group, and the Hertz Foundation.